Mic IVD - Registered and Ready to Deploy
The world’s first magnetic induction cycler is now a registered medical device with CE-IVDR and TGA approval. Mic IVD is manufactured under an ISO 13485:2016 Quality Management System.

Get accurate results, quickly and easily, in the lab or on-the-go.
Never be frustrated with your results again. Engineered in our facility in Australia, the Mic IVD qPCR has served as an indispensable instrument for our partner facilities across the US, Europe, Africa, Latin America, and Asia. Integrating this instrument into your workflow will enable you to gather data and make a clinical diagnosis seamlessly.

Feel Confident In Your Results
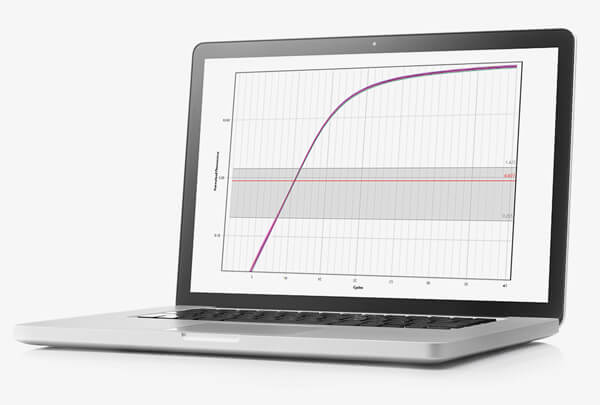
The Mic delivers unrivalled reproducibility between samples, runs and instruments so you can be confident in your results.

Get Results In Less Time
If COVID-19 has taught us anything, it’s that time is critical during an outbreak. Completing runs in under 40 minutes is the new standard with Mic, not the exception. Maintaining assay performance even at speed ensures you can trust that data.


Intuitive Software for Easy Use
Still the same plug-and-play format, with IVD features including CFR 21 part 11 traceability, export functionality to LIMS, user permission levels and full validation as required by a medical device.

Molecular Diagnostics Anywhere, Anytime
It’s a new world of diagnostics after COVID-19. Speed and portability are essentials not conveniences. At 2kg this is the most portable and compact qPCR IVD on the market. No servicing required.
TGA and CE-IVDR
The Mic IVD instrument is intended to be used with CE-IVDR registered clinical diagnostic qPCR kits to provide detection of nucleic acid sequences in human-derived specimens. The Mic IVD instrument is intended for in-vitro diagnostic use by trained laboratory technicians and pathologists to interpret the results to make a clinical diagnosis.


Mic IVD can be sold in the following countries:
Operating in Australia for over a decade, BMS is a leading provider of innovative lab equipment and instruments. We’ve served clinical facilities from all over the globe so rest assured that we can support your facility no matter where you’re located.
Europe
Austria, Bulgaria*, Belgium, Cyprus, Estonia#, France, Germany, Iceland*, Ireland, Italy, Latvia*, Liechtenstein, Lithuania#, Luxembourg, Malta, Netherlands*, Norway*, Poland*, Portugal, Slovenia*, Spain, Switzerland (CE-IVDR)
UK (MHRA)
Türkiye (TiTCK)
*English is an accepted language by the user (for professional use).
Asia-Pacific
Australia (TGA)
Indonesia (MoH)
Iran (IMED)
Japan (PMDA)
Korea (MFDS)
Malaysia (MDA)
North and South America
United States (FDA)#
Brazil (ANVISA)
Canada (Health Canada)#
Uruguay (MSP)#
Colombia (INVIMA)
#Coming Soon

Mic IVD | Specs

Front

Back
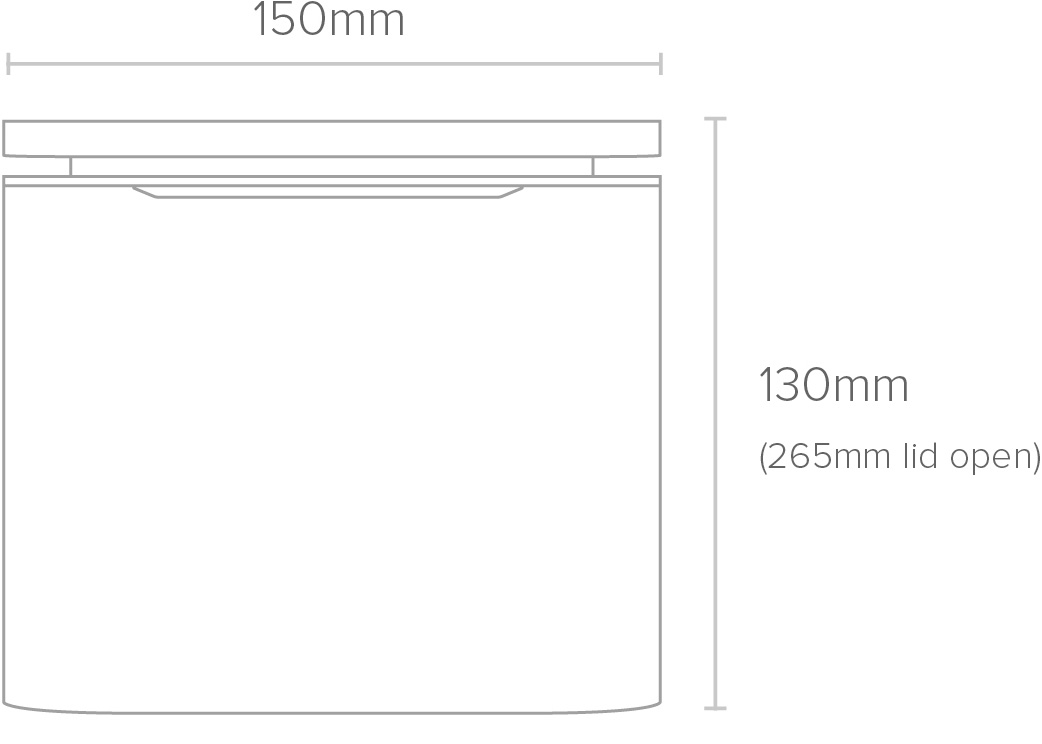
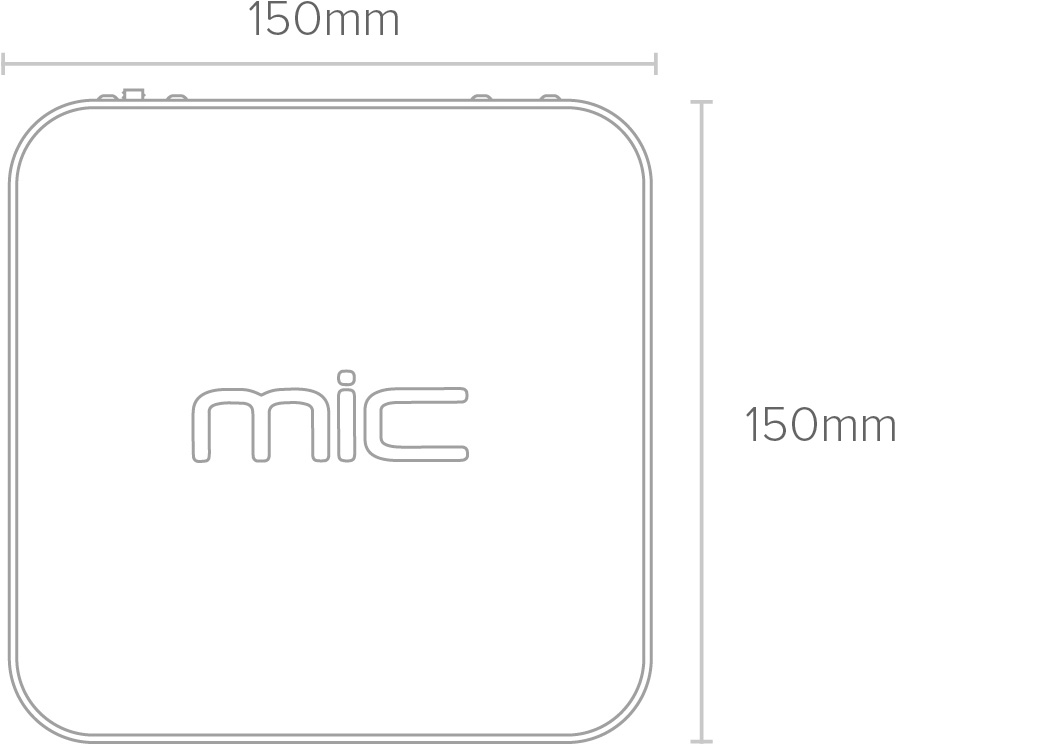
Physical
| Height | 130mm (265mm lid open) |
| Width | 150mm |
| Length | 150mm |
| Weight | 2 kg |
 |  |
Thermal Performance
| Temperature Accuracy | ±0.25°C (60 – 95°C) ±0.50°C (otherwise) |
| Temperature Uniformity | ±0.20°C |
| Ramp Rates | Heating 4°C/s (min.) Cooling 3°C/s (min.) |
| Temperature Input Range | 35 – 99°C (min. 40°C when cycling) |
Optical
| Detectors | Photodiode per channel | ||
| Excitation Sources | High power LED for each channel | ||
| Channels | Green | Ex 465nm | Em 510nm |
| Yellow | Ex 540nm | Em 570nm | |
| Orange | Ex 585nm | Em 618nm | |
| Red | Ex 635nm | Em 675nm | |
| Acquisition Time | 1 second | ||
Reaction Tubes
| Samples per Instrument | 48 |
| Reaction Volume Range | 5 – 30µL |
Operating Environment
| Temperature | 18 – 35ºC |
| Relative Humidity | 20 – 80% |
Transport and Storage Conditions
| Temperature | 5 – 35ºC |
| Relative Humidity | 0 – 95% (no condensation) |