Kinases
Kinase enzymes are at the heart of cellular communication, playing a pivotal role in translating external signals into functional responses within cells. Their involvement in cell growth, division, and death makes them key targets in the development of therapies for diseases such as cancer, where these processes are dysregulated. The need to understand kinase activity for therapeutic purposes has given rise to the development and refinement of kinase assays in drug discovery.
Accurate measurement of kinase activity depends on the addition of reagents, substrates, and inhibitors at specific concentrations, volumes, and times. The Myra liquid handling system has been shown to provide unmatched precision in liquid dispensing, reduced manual intervention, and minimized human errors, revolutionizing the conduct of kinase assays.
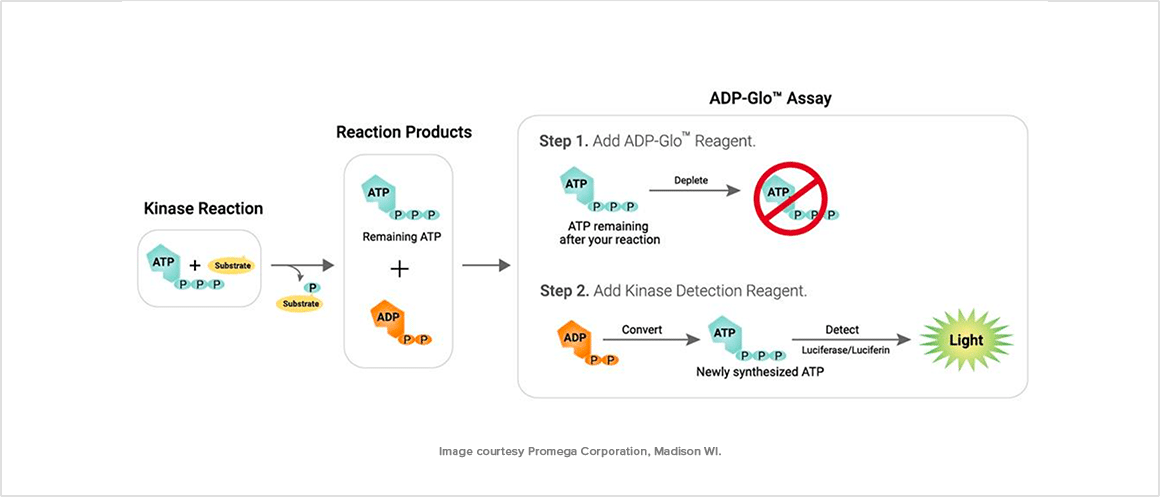
ADP-Glo
The ADP-Glo Kinase Assay is a luminescent assay that measures the amount of adenosine diphosphate (ADP) produced by kinase reactions over time.
It is used to evaluate the activity of kinase enzymes and to screen for kinase inhibitors by quantifying the conversion of ATP to ADP in a time-course manner, making it a valuable tool for drug discovery and development programs targeting kinase-driven pathways.
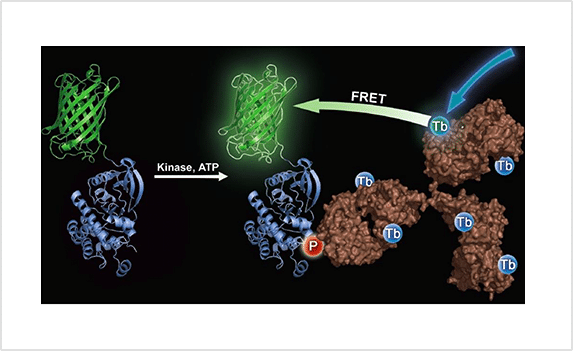
TR-FRET
Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET) is a sensitive and reliable fluorescence-based assay technique that combines the principles of time-resolved fluorescence and FRET to minimize background signal and maximize signal-to-noise ratio. It is widely used in biochemical and biological research for studying molecular interactions, such as protein-protein or protein-DNA interactions, enabling the detection and quantification of these interactions in various assay formats, including kinase large molecule high throughput screening for drug discovery.
Kinase Assays on Myra
Browse a comprehensive table of all available templates on Myra, complete with direct links to specific MyraScript pages. Access protocol documentation via a dedicated PDF link. Verified options include templates for BMS, Partner (kit company), and the Myra community (customers).
| Kit | Verified By |
|---|---|

| BMSCommunity |
Case Study: Promega ADP-Glo Kinase Assay
Reliable, Reproducible Kinase Inhibitor Screening
ADP-Glo Kinase time course assay was run to determine the enzyme kinetics of a kinase with a peptide substrate.
In a typical ADP-Glo™ Kinase Assay, the process starts with the kinase reaction, where a kinase enzyme phosphorylates a substrate in the presence of ATP. Following this, the ADPGIo™ Reagent is added to stop the kinase reaction and deplete remaining ATP, preparing the system for ADP measurement.
Finally, the Kinase Detection Reagent converts ADP back to ATP, which is quantified through a luminescent signal.
Automating this sequence with Myra ensured that each step was precisely timed and executed, maintaining the integrity and consistency of the assay across multiple wells and plates.
Experimental Setup
Small volumes done really well.
DP-Glo Kinase Assay was conducted using peptide diluted 1:2 in a total volume of 2 mL. The peptide was diluted across the plate followed by the addition of 2 mL of kinase. Four microliters of a stop solution were added at 5 min intervals from time zero.
The systematic addition of components needed to take into account the specific times required to ensure accurate addition of the stop solution. An ADP standard curve was created in triplicate to allow for ATP to ADP conversion calculations.

Results
High quality data.
Myra facilitates the generation of data sets necessary for Michaelis-Menten analysis by ensuring that reaction conditions are consistently replicated. This uniformity allows for the precise determination of reaction velocities at varying substrate concentrations, critical for plotting Michaelis-Menten curves and calculating kinetic parameters.
Kinase large molecule high throughput screening on Myra enables rapid and reproducible analysis of numerous inhibitors, accelerating the identification of promising therapeutic candidates. Integrating with a q-PCR Machine further supports downstream validation, connecting biochemical screening data to gene expression or cellular outcomes.
