As the world opens up over the coming months and years, there will be countless plane journeys to see loved ones, meet new family members and travel for work and leisure. Many of these travellers will first need to provide evidence of a negative PCR test to show they pose a minimal COVID-19 transmission risk. Time is proving of the essence for testing.
With reports of multi-hour queues at airports from Portugal to India to Britain because of these important testing requirements, it’s clear that test efficiency is paramount. Delays could lead to missed flights, extra testing and logistical nightmares on a grand scale – particularly so in the world’s busiest terminals! Queues themselves could become points of transmission if someone happens to be infectious. Fortunately, accurate and efficient PCR testing is entirely possible with the right approach.
Firstly, why are PCR tests required at many airports?
While every country has its own requirements for COVID-19 border control, many now require a negative polymerase chain reaction (PCR) test result within a certain time of each inbound passenger taking off on their flight. There are rapid antigen diagnostic tests available for COVID-19 – so why not use these instead? As we covered in a previous article, real-time PCR molecular testing is considered the gold standard testing method because it has greater accuracy with higher sensitivity and specificity to viral loads. Rapid antigen tests aren’t as effective in detecting low levels of the virus at the beginning or tail end of someone’s infectious period. Less accurate testing could allow new and unwanted strains of COVID-19 to make their way across borders and infect new populations.
As such, PCR is considered the top choice for important applications such as plane travel. It’s also been traditionally slower than rapid antigen testing, because PCR requires a lab setting – that is, until recent years. New PCR technologies such as Mic and Myra are supporting both rapid and accurate testing at key COVID-19 management points.
How airports are investing in timely PCR testing
As airport travellers increase, diagnostic laboratory providers have been establishing networks of bespoke on-site testing laboratories at major UK airports. These on-site PCR testing facilities require:
- Efficiency, to meet demanding turnaround times
- Scalability, to meet varying and increasing demand, and
- Accuracy, to support vital COVID-19 management across borders. In particular, these on-site facilities would need to be highly accurate when used with CE-IVD marked assays available from commercial suppliers.
It’s for these reasons that multiple providers have selected the Mic qPCR cycler as their system of choice, with over 150 of the cyclers now in use for testing across the United Kingdom. These unique thermal cyclers can complete runs in as little as 40 minutes using magnetic induction technology, which offers excellent accuracy as well as reproducibility across up to 10 machines linked to the same computer.
Another traditionally labour-intensive step has been the master mix preparation, distribution and addition of samples to the PCR reaction tubes. The chosen solution has been the Myra liquid handler, which is fully compatible with traditional 96- and 384-well PCR systems and integrates with the Mic qPCR Cycler. This means sample data is transferred seamlessly between the liquid handling system and cycler for significantly faster PCR results.

Multiple Mic cyclers and Myra liquid handlers set up to ready to screen passengers at Liverpool Airport.


Mic and Myra in use on the bench.
A look at the numbers
Let’s take a look at an example workflow to see how this efficiency can translate in the real world.
| Workflow Step | Approximate Timing |
| Sample Prep using Direct Lysis Reagent e.g. Microlysis, QIAprep
| 20 minutes
|
| Preparation of 48 Mic qPCR Tubes Automated on Myra Liquid Handling System
| 12 minutes |
| Rapid CE-IVD qPCR Assay Running on Mic qPCR Cycler e.g. geneMAP™ 2019-nCoV
| 45 minutes
|
| Automatic qPCR Analysis using Identifier mode Results presented to user on completion of the run
| 0 minutes
|
| Total
| 1 hour, 12 minutes |
So within an hour and a half of a traveller’s nasal swab being taken, the result could potentially be delivered to the traveller and to airport authorities. This is a marked improvement on manual processing and traditional cyclers, which might take 4-6 hours of processing per sample. Within a busy airport this can save countless hours and logistical headaches, each and every day.
What does data look like within this PCR ecosystem? Mic can display cycling data in various formats as is required. CE-IVD marked assays typically present their result calling criteria in terms of Cq values within a multiplex reaction (also referred to as Ct or Cp – the point at which the PCR curve crosses the pre-set Threshold). In the case of a COVID-19 assay, an example configuration is as follows.
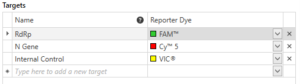
qPCR Target List as displayed in Mic qPCR software.
Here two targets are used to identify the pathogen (RdRp and N Gene in this case), and a third channel used to check the success of the PCR reaction (Internal Control).
If we look closer at cycling analysis, specific settings to interpret each channel can be saved into the software by the user to be applied consistently on completion of every run.
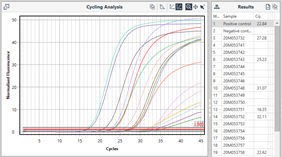
Example cycling analysis from Mic qPCR, with Cq values presented.
Cq values can then be manually compared with the validated criteria provided by the assay manufacturer, to create a result call for each sample.
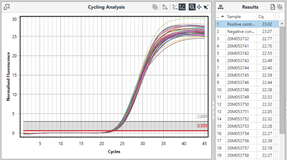
Automating data into results using Identifier analysis
For even greater efficiency, this final manual comparison step can be automated using another mode of the Mic software called Identifier. This takes the Cq values of each channel generated by the Cycling analysis, and applies a rule set either validated by the assay manufacturer or specified by the user, to generate a results table – the final product of a qPCR workflow.
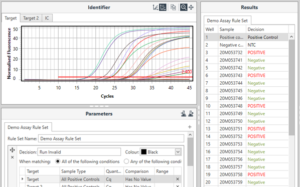
An example Identifier results table, providing simple negative or positive results.
This mode can also be configured to apply automatically on completion of each run, offering additional options for standardisation within the lab. This functionality can save precious minutes on each run and allow results to be delivered quickly and easily.
What this all ultimately means for airports and other testing
Logistical efficiency within an airport or similar location shouldn’t mean foregoing effective COVID-19 management. There should be no need to compromise with lesser testing methods. By choosing the right technology, an ideal balance between accuracy and efficiency can be maintained.
We’d love to help you experience the benefits of Mic and Myra for your own lab or testing site. For further information or to arrange for an onsite demo at your convenience, you can contact us at sales@biomolecularsystems.com or call your local distributor today.